In the second part of VIGILINT’s two-part series COVID-19 1 Year Later, VIGILINT Ops Analyst and blogger Colin Schilly dives into the two critical hallmarks of the pandemic. Testing and vaccines. The development and rollout of several effective vaccines in under a year offer great hope for the pandemic’s end. Sadly, that day may still be far off. Experts agree that even with vaccines and an aggressive immunization distribution campaign from President Biden, widespread testing is critical for turning the tide against COVID-19.
COVID-19 Testing: Development and Distribution
To navigate a pandemic, officials must understand disease prevalence in the community. Disease prevalence is the total number of disease cases in a population, usually per 100,000 people. This number informs local and regional testing policies, social distancing requirements, public health measures, and reopening timelines. Ideally, disease modelers and governments use data collected from sample populations tested for real-time infection (molecular testing) and past infection (antibody/serological testing). The United States mostly failed to fulfill this fundamental pandemic response requirement, as increased demand for tests repeatedly overwhelmed supply.
A Slow Start
Officials confirmed the first case of COVID-19 outside of mainland China on January 13, 2020, in Thailand. Six days later, scientists investigating the virus informed the WHO of the type of tests needed to detect the virus. The WHO immediately began assembling kits and distributing kit instructions to nations worldwide. Opting not to use the WHO test, the CDC quickly announced they would create their own tests. The CDC had its first COVID-19 kits ready on January 28 and received an Emergency Use Authorization (EUA) from the FDA on February 4. Distribution commenced the following day.

It did not take long for scientists to notice an error in the ingredients within the CDC kits, making interpreting test results impossible. On February 12, the CDC advised all labs to halt the production and processing of their kits. While other government-funded labs and testing companies could conduct tests, the CDC was the only group authorized to create diagnostic COVID-19 tests at the time.
Not until February 29 did the FDA allow labs that developed and validated their own tests to submit for a EUA. While many states continued to rely on CDC test kits, laboratories nationwide began producing their own diagnostic tests. More companies making more tests opened the playing field, but labs suffered supply change issues and resource shortages due to the increased COVID-19 cases around the country. Diagnostic testing is such an integral part of managing the spread of infectious diseases that the initial failure of a coherent COVID-19 testing system has plagued the US response ever since.
Strict Requirements
Once the FDA approved several different tests for COVID-19, companies ramped up production to rates never before seen. As supply began to catch up with demand, however, another problem quickly arose: many people did not qualify to get a test. States had rigorous requirements, usually reserving tests for healthcare workers or those with moderate to severe symptoms. Restricting tests was one way to preserve PPE (personal protective equipment) as medical professionals would need PPE to administer every test. The lack of widely available tests allowed the virus to spread silently. Estimates have shown that 17%-20% of COVID-19 infected patients are asymptomatic throughout their infection.
A Balancing Act
The last, most persistent problem is supply—not just the amount of tests, but everything that goes with them. Early on, companies struggled to produce enough tests to satisfy a worried public. Companies eventually caught up, but demand exposed various deficiencies in the supply chain. In November, testing facilities nationwide faced a renewed wave of tests ahead of the holiday season. At this point, “consumable supplies” like pipettes and swabs were running out. The American Society for Microbiology found that 134 clinical labs reported operating at an average of 50.8% capacity during this time. Meanwhile, demand for tests from companies like Quest Diagnostics was up 50%.
It doesn’t take an economist to recognize the inherent balancing act of supply and demand. As companies raced to produce tests, they devoted resources to the production of reagents and test kits. Depletion of consumable supplies then caused problems months later. Even with adequate supply, processing tests require around-the-clock labor, and trained lab technicians cannot work 24/7. Companies continue to struggle with these problems and will likely face another wave of testing when people return to work.
Moving Forward
On November 17, the FDA issued a EUA for the first test kit that is entirely self-administered and provides rapid results. The test is currently authorized for prescription use only but could help ease the testing facilities’ logistical burden. While far from perfect, testing development and distribution has become more efficient with time and will continue to improve.
Vaccines: Development and Distribution
Vaccines are one of the greatest success stories in the history of modern medicine. The development of these treatments has lead to the eradication of smallpox and nearly eliminated polio. We know that the key to beating the COVID-19 crisis is the success of vaccine treatments and herd immunity, which is why the development of not one but two 95% efficacy vaccines in under a year is a tremendous scientific achievement.
Vaccine Development
On January 11, 2020, the WHO received the genetic sequence of SARS-CoV-2 from Chinese scientists. Since then, researchers around the world have devoted themselves to the development of a vaccine.
Programs like America’s Operation Warp Speed (OWS) poured money into vaccine development and production as early as March. Purchasing and manufacturing guarantees mitigated production’s financial risk, allowing companies to mass-produce different vaccine types without compromising safety. Worldwide collaboration, massive funding, and mRNA technology contracted vaccine development timelines considerably, enabling companies to accomplish in months something that would typically take years. In the United States, vaccines from Pfizer-BioNTech, Moderna, Johnson and Johnson, and Oxford/AstraZeneca will prove crucial to reaching herd immunity.
As of February 4, 2021, the US has distributed 62.8 million doses of COVID-19 vaccines and administered 43.2 doses. At this pace, 75% of the population will receive the first dose by November 2021.
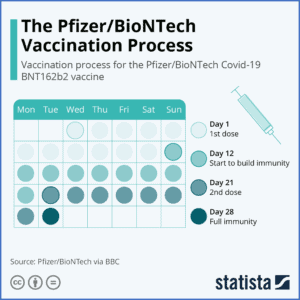
Pfizer/BioNTech
Production of the BioNTech vaccine, officially named Comirnaty, began in January 2020. The German company partnered with the American drug producer Pfizer to increase production capacity in March. While not a part of OWS, the company received a $1.9 billion contract from the US in July for 100 million doses. A later agreement guaranteed an additional 100 million. They began Phase III trials in July and published their first results on November 9, showing 95% effectiveness – a remarkable scientific achievement. Pfizer-BioNTech’s candidate became the first vaccine approved by the FDA on December 11, starting the US vaccine rollout process.
Comirnaty requires storage at -94°F due to its use of mRNA, which presents a significant distribution problem. The vaccination also requires two doses, administered three weeks apart, which has caused logistical challenges.
Moderna
Moderna also began production in January and was the first company to begin human trials in March. A deal through OWS granted the Boston company nearly $1 billion in support. A second deal in August for $1.5 billion guaranteed 100 million doses, with an option for an additional 100 million in the spring of 2021. Phase III results, released on November 16, indicated 94.1% effectiveness, and the FDA issued a EUA on December 18.
Moderna’s vaccine is the only other candidate to use mRNA technology but can be stored at -4°F, closer to freezer temperature. Its approval in the US eased distribution pressure slightly, but its two-dose requirement has still contributed to delays.
Johnson and Johnson
The FDA announced that it would convene on February 26 to discuss the EUA application by Johnson & Johnson and Janssen Biotech, Inc. The authorization would be a significant lift in the vaccine rollout as the vaccine candidate from the New Brunswick, New Jersey-based company has substantial advantages over the current EUA vaccines. Unlike Pfizer and
Moderna, Johnson and Johnson’s candidate uses a modified adenovirus and can be refrigerated for up to three months. Just as important, it only requires one shot for full administration. 45,783 participants participated in the Phase 3 trials. Effectiveness against moderate to severe COVID-19 cases is 72% in American participants. The trial data suggests 85% efficacy against severe COVID-19.
On February 11, 6,500 pharmacies are expected to administer 1 million doses of the Johnson & Johnson vaccine every week.
Johnson and Johnson have received nearly $1.5 billion in funding from the US, with a deal for 100 million doses if approved. The company plans to produce one billion doses in 2021.
Oxford/AstraZeneca
While already authorized in the UK and elsewhere, the FDA will not approve the Oxford/AstraZeneca for some time. Researchers published Phase III results on December 8, but the FDA will not authorize the vaccine without results from US-based trials, expected around April.
An agreement made with the US in May guaranteed 300 million doses once the vaccine is approved. As the vaccine lasts six months in a refrigerator, this EUA would provide a crucial supply boost for the US.
Moving Forward
Much of the US health system’s problems with COVID testing have come up with the vaccine rollout. It takes time to streamline distribution and administrative protocols to maximize both safety and efficiency.
President Biden has purchased 600 million doses and aims to administer 1.5 million doses per day. The US is currently averaging around 983,000 shots per day. It would take about 18 months to reach herd immunity at the current rate, which means vaccinating 70%-80% of the population.
Distribution success will depend mainly on federally run vaccination clinics, also advocated by the Biden administration, and new vaccine authorizations. The White House is reportedly close to deals with Pfizer and Moderna for an additional 100 million doses each, expected by the end of summer. In addition to the current supply, these doses would enable the vaccination of 300 million people. Estimates vary when the public can be vaccinated; however, most experts agree it will be between late spring and early fall.
