On Sunday, August 23, the US Federal Food and Drug Administration (FDA) announced an Emergency Use Authorization (EUA) for convalescent plasma as a treatment for hospitalized COVID-19 patients citing “known and potential benefits of the product outweigh the known and potential risks of the product.”
The EUA would effectively enable physicians to treat COVID-19 patients in the hospital with convalescent plasma – the blood of recovered COVID-19 individuals containing the SARS-CoV-2 antibodies. However, the FDA noted randomized trials “remain necessary to determine the optimal product attributes and appropriate patient populations for its use.”
Criticism of the FDA’s EUA
As part of the FDA’s announcement, Alex Azar, US Health and Human Services Secretary, referenced a Mayo Clinic study released on August 12 that 35,000 patients who were “treated with plasma containing the highest levels of antibodies had a 35 percent lower risk of dying compared to those treated with less-rich plasma.” The study and Sunday’s remarks have been criticized for scientific limitations and “misrepresentation of the data.”
Dr. Scott Gottlieb, a former FDA commissioner said that convalescent plasma met the FDA standard for EUA, and noted it could be beneficial or “it might be weakly beneficial. It doesn’t look like a home run, but right now we are looking for singles and doubles.” He continued, “There aren’t really going to be any home runs on the horizon until we get the other therapeutic antibodies on the market and hopefully eventually vaccines and better therapeutics.”
Since the FDA announcement, members of the scientific and public health community have criticized the agency and its commissioner, Dr. Stephen Hahn, for authorizing the treatment without more rigorous and randomized controlled trials. It should be stated that of the 35,000 patients in the Mayo Clinic study, 49.8% were also treated with steroids and 30.1% were treated with Remdesivr – an antiviral medication also being used to treat COVID-19 – thus making it difficult to ascertain the sole effectiveness of convalescent plasma as a treatment for COVID-19.
What is the Process of Convalescent Plasma?
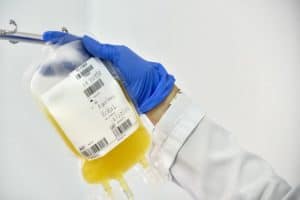
If you had not heard of the medical term “antibodies” before the pandemic, chances are you have by now. COVID-19 antibody testing, or serological testing (covered in VIGILINT’s Medical Bulletin # 14), is a blood sampling process that detects the presence of neutralizing SARS-CoV-2 antibodies (the virus causing COVID-19 disease) thereby revealing if an individual has had a COVID-19 infection, whether symptomatic or asymptomatic. These antibodies may provide a layer of immunity for future COVID-19 infections, but the amount of protection and the duration of that immunity is still unknown. Beyond potentially protecting the individual from future COVID-19 infections, antibodies remain a critical research component for viable treatment options.
Convalescent is a term referring to a person recovering from an illness. Antibodies float in the blood plasma – the yellow, liquid component left after blood is stripped of red and white cells. Medical professionals “harvest” the plasma from donated blood, perform tests and then purify and isolate the SARS-CoV-2 antibodies. Acutely-ill COVID-19 patients can then be treated with this convalescent plasma to hopefully boost their ability to fight the virus.
While there is a fierce agreement in the health care community that more trials need to be conducted, convalescent plasma has shown some promise in the fight against COVID-19. In order to prove, or disprove its effectiveness, there is one other critical point where the community is in agreement: the need for more convalescent plasma donors.
Some recovered COVID-19 individuals may naturally have different amounts of antibodies than others who have also recovered, based on how their immune system responded to the virus and the duration of their illness. It is essential that a variety of convalescent plasma be used to attest to the different levels of antibodies a patient receives and how that impacts recovery.
Rachel Daniel, Manager at BPL Plasma, a plasma collection center based in Wilmington, North Carolina stated:
“On April 30, 2020, BPL launched a COVID-19 convalescent plasma collection program to help in the development of lifesaving plasma-derived treatments for those battling COVID-19. We are seeking help from those that have recovered from COVID-19, they are vital to helping us find a treatment. Convalescent plasma has been used to create therapeutics for diseases such as tetanus, hepatitis B, and rabies for many years.”
Individuals who have fully recovered from COVID-19 for at least 14 days are encouraged to donate plasma.
