-
- In what is considered the most comprehensive global study of bacterial antimicrobial resistance (AMR), the peer-reviewed Lancet Medical Journal found that nearly 5 million deaths in 2019 were associated with antibiotic-resistant bacterial infections.
- In addition, the study found that drug-resistance bacteria accounted for 1.2 million deaths in 2019 – more than HIV and malaria.
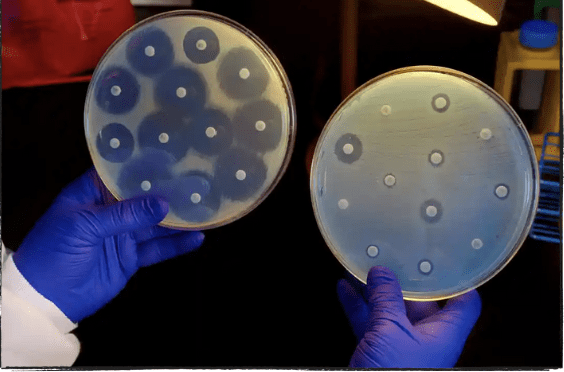
Antibiotic Resistance
The World Health Organization (WHO) considers antibiotic resistance “one of the biggest threats to global health, food security, and development today.” Bacteria and fungi-fighting drugs, which we know as antibiotics and antifungals, are becoming increasingly less effective in killing the bacteria they are designed to attack.MalariaVaccineDiseases and infections like pneumonia, blood poisoning, UTIs, gonorrhea, and foodborne illnesses are becoming more challenging or impossible to treat. The drugs developed to kill them cannot do the job like they are supposed to anymore.
Researchers from the Lancet study, which looked at 471 million records from 204 countries and territories, found that resistant pathogens or “superbugs” are “mutating to evade antibiotics at a more rapid pace than previously forecasted.”In the US, 2.4 million infections and 35,000 deaths are estimated to occur each year because of AMR, affecting individuals of all ages, race, and economic statuses. Sadly, AMR is now one of the leading causes of death worldwide.
How Antibiotic Resistance Works
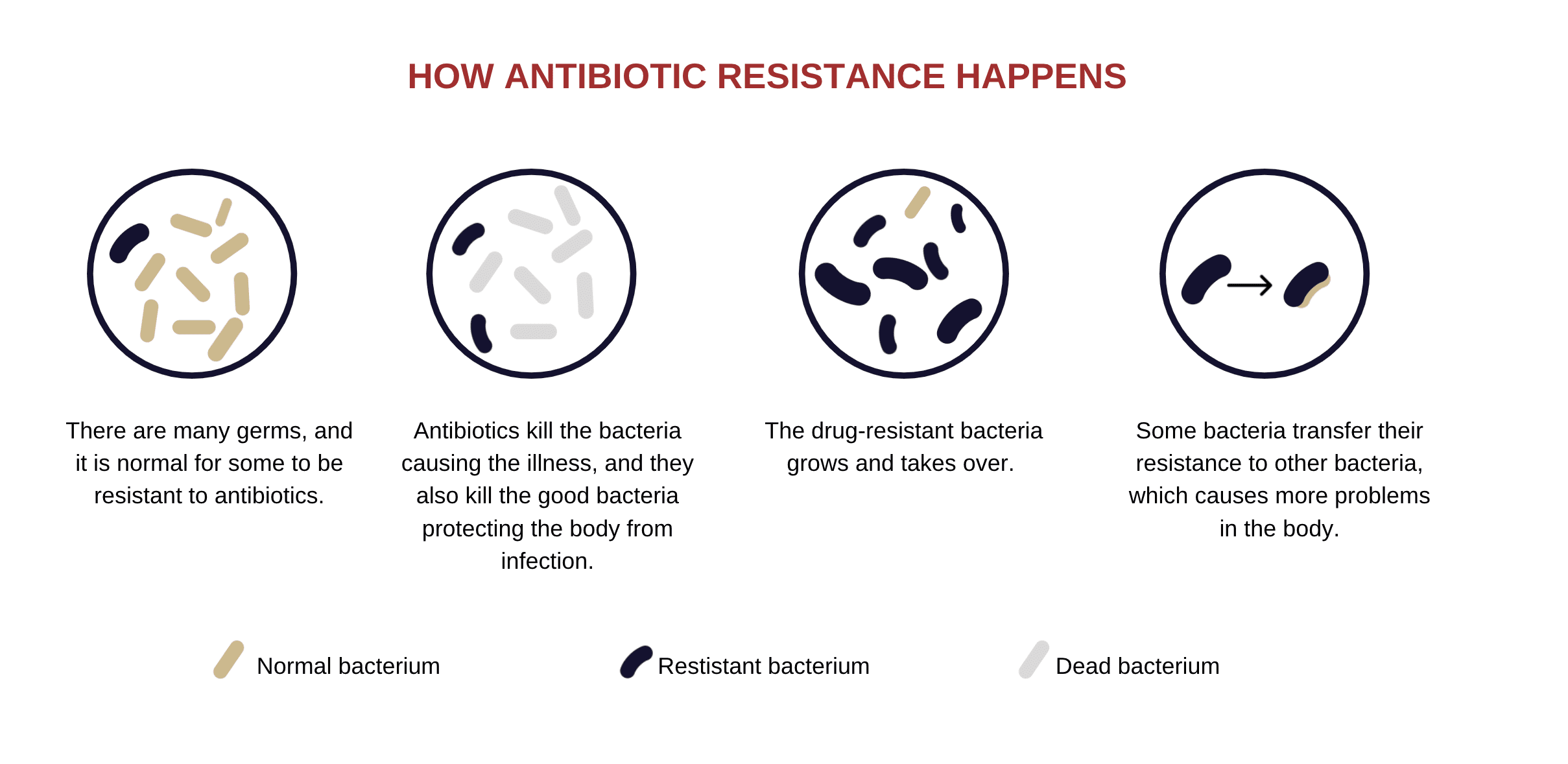
According to Chris Murray, the director of the Institute for Health Metrics and Evaluation and one of the authors of the Lancet study, “The more we use the antibiotics, the more likely we are to see resistant pathogens spread.”
Medical personnel in many countries have prescribed antibiotics in an attempt to prevent secondary bacterial infections (like a sinus infection) but do so prematurely, or are giving in to patient demand.
In a study published by the Oxford Academic in 2019, patient demand was the most common reason clinicians gave for prescribing non-indicated antibiotics. The motivations to do so were related to “concerns about providing value for the patients, fear of negative repercussions from denying antibiotics, and the approach to inconvincible patients.”
The Effects of Antibiotic Resistance
As bacteria become more and more resistant to antibiotics, the more severe previously antibiotic-treatable illnesses will become. As a result, common infections and minor injuries could result in more complicated and prolonged sickness, higher medical costs, extended hospital stays (putting strain on hospital systems), and increased mortality risk.
According to the Lancet study, pneumonia and other lower respiratory infections accounted for 400,000 AMR-related deaths. Additionally, E. coli and MRSA (methicillin-resistant Staphylococcus aureus) are the two drug-resistant pathogens that accounted for the most deaths.
The inability to fight infections is a primary concern for scientists, but there are other medical settings where antibiotic resistance is an urgent public health concern. From the CDC: “Many medical advances are dependent on the ability to fight infections using antibiotics, including joint replacements, organ transplants, cancer therapy, and the treatment of chronic diseases like diabetes, asthma, and rheumatoid arthritis.”
How We Can All Help
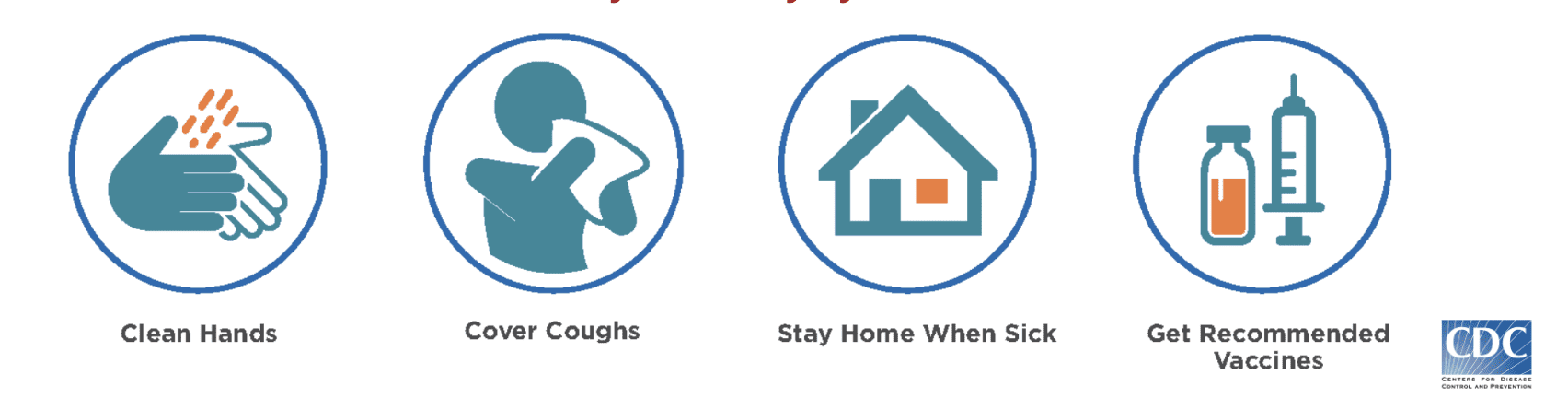
Individuals, health care providers, and policymakers all have a role in reducing the global burden of AMR. Individuals should only take antibiotics when absolutely necessary and should not pressure providers when they state that drugs are unnecessary. As we learned from COVID-19, personal hygiene is key to reducing the risk of infection and disease – washing hands, washing food during preparation, and avoiding contact with sick individuals.
Medical professionals should only prescribe medications when needed and stay up-to-date on current protocols and guidelines for dispensing them. If patients push back, physicians need to educate them on the harm antibiotics can do when not needed and highlight ways patients can avoid infections through safe hygiene. Medical providers should always report AMR infections to the proper public health channels.
Policymakers need to develop and implement a thorough plan to tackle antibiotic resistance and improve antibiotic prescribing and use. The WHO advocates for policymakers to create more robust surveillance of antibiotic-resistant infections, and health care providers only prescribe antibiotics when necessary.
Be Antibiotics Aware
The FDA approved several new antibiotics that can treat certain resistant bacteria and advocated for additional clinical trials for other drugs developed in the US. Additionally, the FDA and the CDC co-developed a public health campaign named “Get Smart: Know When Antibiotics Work,” designed to educate prevention of antibiotic-resistant infections.
Eric Ossmann, VIGILINT’s Chief Medical Officer, advises individuals to practice good hygiene and remain up to date on vaccinations. When traveling, it is essential to note which infectious diseases are prevalent in those areas and take precautions to avoid transmission as much as possible, thereby reducing the need for antibiotic treatment